Abstract
Background: A current focus of CML research is achievement of sustained, deep molecular response (DMR) on TKIs, with the goal of stopping therapy. In clinical trials of TKI discontinuation, approximately 50% maintain their response (Saussele. Leukemia. 2016). Dasatinib is a suitable option for pts considering treatment-free remission (TFR) because it induces high rates of early, deep, and sustained responses (Cortes. JCO. 2016). Here, we report results from all pts enrolled in the phase 2 DASFREE study, investigating TFR in the largest population of pts discontinuing dasatinib in the 1st line and beyond.
Methods: DASFREE (CA180-406/NCT01850004) is a phase 2, open-label, single-arm study in adults with CML-CP on dasatinib for ≥2 yr as 1st-line or subsequent therapy. Eligible pts also had dasatinib-induced DMR (MR4.5 or BCR-ABL1 ≤0.0032% on the International Scale) confirmed at a local lab for ≥1 yr prior to enrollment, with a 1-log reduction in BCR-ABL1 from baseline within 3-6.5 mo of starting dasatinib. During the screening phase, MR4.5 was confirmed at a central lab twice within 3 mo prior to dasatinib discontinuation. BCR-ABL1 was monitored centrally after discontinuation every mo in the 1st yr, then every 3 mo. If major molecular response (MMR) was lost, pts resumed dasatinib at the previous dose. The primary endpoint is rate of MMR 1 yr after dasatinib discontinuation. Secondary endpoints include BCR-ABL1 kinetics, event-free survival (EFS; no loss of MMR), relapse-free survival (RFS; no loss of MMR, complete cytogenetic response, or complete hematologic response, or progression to accelerated/blast phase [AP/BP] CML), rate of transformation to AP/BP, progression-free survival, and overall survival. Exploratory analyses include frequency of adverse events (AEs) after discontinuation and during dasatinib treatment, and MMR after reinitiating dasatinib. This analysis includes assessment of the entire cohort of 84 enrolled pts as of July 2017 (minimum follow-up of 6 mo). Analysis of data for all pts with ≥12 mo of follow-up will be presented.
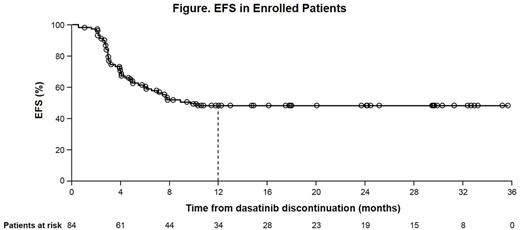
Results: As of October 2016, 84 pts were enrolled (Sokal scores: 63% low, 29% intermediate, 6% high, 2% unknown; no pts had prior interferon; 38 pts on 1st-line dasatinib). At the time of this analysis, 63 pts had ≥12 mo of follow-up after discontinuation. Median dasatinib dose at discontinuation was 100 mg/d (range 20-150). EFS at 1 yr was 49% (95% CI 37, 59; Figure). Forty-three of 84 pts (51%) lost MMR. Median time from discontinuation to loss of MMR was 4 mo (range 1-10). All 43 pts who lost MMR restarted dasatinib, 41 (95%) of whom have regained MMR after re-treatment (1 pt withdrew from study and was lost to follow-up, 1 pt does not have sufficient follow-up). Median time to regain MMR was 2 mo (range 1-4). Median time from CML diagnosis to discontinuation was 72 mo (range 29-156) in pts without loss of MMR after discontinuation and 69 mo (range 29-244) in pts with loss of MMR after discontinuation. Pts maintaining MMR had a median time of 72 mo (range 28-154) on prior TKIs; pts losing MMR had a median time of 67 mo (range 28-221) on prior TKIs. Median time on prior TKIs was shorter in pts treated with 1st-line dasatinib: 46 mo (range 28-89) in pts who maintained MMR and 34 mo (range 28-87) in pts who lost MMR. In a multivariate analysis, no relationship was found between RFS and duration of prior TKI and line of therapy. No transformation events or deaths were observed. Most AEs occurred off-treatment, although not all were attributed to withdrawal events. Musculoskeletal disorders were reported in 23 pts (27%) off-treatment, and in 8 pts (with 14 events) these were considered withdrawal syndrome. Withdrawal events occurred after a median of 3 mo (range <1-6) after discontinuation; 9 of these events resolved after a median of 3 mo (range 1-9). Interestingly, hypertension occurred in 6 pts (7%) off-treatment. There were no AEs leading to discontinuation from the trial.
Conclusions: Data from DASFREE support the feasibility of TFR in pts with CML-CP in DMR treated with dasatinib in the 1st line and beyond. In this largest analysis of pts with CML-CP in DMR who discontinued dasatinib, approximately 50% of pts maintained MMR. With a minimum follow-up of 6 mo, 41 of 43 pts who lost MMR quickly regained their response after therapy was reinitiated. There was a small number of pts with symptoms of dasatinib withdrawal. Data for all enrolled pts with ≥12-mo of follow-up will be presented.
Shah: ARIAD: Research Funding; Daiichi-Sankyo: Research Funding; Bristol-Myers Squibb: Research Funding; Pfizer: Research Funding. García Gutiérrez: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Saussele: Pfizer: Honoraria; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Incyte: Honoraria. Rea: Incyte: Honoraria; BMS: Consultancy, Honoraria; Pfizer: Honoraria; Novartis: Consultancy, Honoraria. Mahon: PFIZER: Consultancy, Honoraria; INCYTE: Honoraria; BMS: Consultancy, Honoraria; NOVARTIS PHARMA: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Levy: Actinium Pharmaceuticals: Equity Ownership; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding; Takeda: Consultancy, Speakers Bureau. Gómez-Casares: BMS: Speakers Bureau; Incyte: Speakers Bureau; Pfizer: Speakers Bureau; Novartis: Speakers Bureau. Pane: Novartis: Honoraria, Speakers Bureau. Nicolini: Incyte Biosciences: Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Research Funding. Mauro: Bristol-Myers Squibb: Consultancy. Sy: Bristol-Myers Squibb: Employment. Martin-Regueira: BMS: Employment. Lipton: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal